ARL892 13965-03-2
Note: For research use only, not for direct human use.*
| Pack Size | Unit Price | Stock in Mumbai- WH | Stock in Hyderabad- WH | Quantity |
|---|
Description : Palladium(II) acetate (Pd(OAc)₂) is a widely used catalyst in organic synthesis, known for its efficiency in cross-coupling and oxidation reactions. ARL offers high-quality, nitro-free Palladium(II) acetate catalysts, ensuring superior performance and consistency in research and industrial applications.
| Appearance | Orange to Brown powder to crystal |
| Purity(Chelometric Titration) | min. 98% |
| Confirms Structure | NMR |
| Melting Point | 205 °C |
| Solubility | Acetone |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | 10°C |
| Conditions to Avoid | Moisture, light and heat sensitive |
| Storage Condition | Store in a cool, dry, and inert atmosphere. |
| Metal content | 47-48% |
| Max. absorption wavelength (UV) | 400 nm (EtOH) |
| Packaging and Container | Glass bottles |
| Shipping Condition | Ambient Temperature |
Pictogram :

|
| Palladium(II) acetate is a chemical compound used in various chemical reactions, but it also presents some safety hazards. It's important to handle it with care, using appropriate personal protective equipment (PPE) and following safety guidelines to minimize risks. Regulations related to its use primarily focus on proper disposal as hazardous waste and avoiding environmental contamination |
Palladium(II) acetate (Pd(OAc)2) is a versatile catalyst in organic chemistry, primarily used in cross-coupling reactions and oxidation reactions. Cross-Coupling Reactions: Palladium(II) acetate is a key catalyst in a wide range of cross-coupling reactions, including: Heck reaction: Formation of carbon-carbon bonds between aryl or vinyl halides and alkenes. Suzuki-Miyaura coupling: Formation of carbon-carbon bonds between aryl/vinyl halides and boronic acids. Buchwald-Hartwig amination: Formation of carbon-nitrogen bonds by coupling aryl halides with amines. Stille coupling: Formation of carbon-carbon bonds between aryl/vinyl halides and tin reagents. Sonogashira coupling: Formation of carbon-carbon bonds between aryl/vinyl halides and terminal alkynes. Negishi coupling: Formation of carbon-carbon bonds between aryl/vinyl halides and organozinc reagents.

ARL892 13965-03-2
ARL6866 246047-72-3

ARL6527 15956-28-2

ARL8632 54010-75-2

ARL8077 14898-67-0
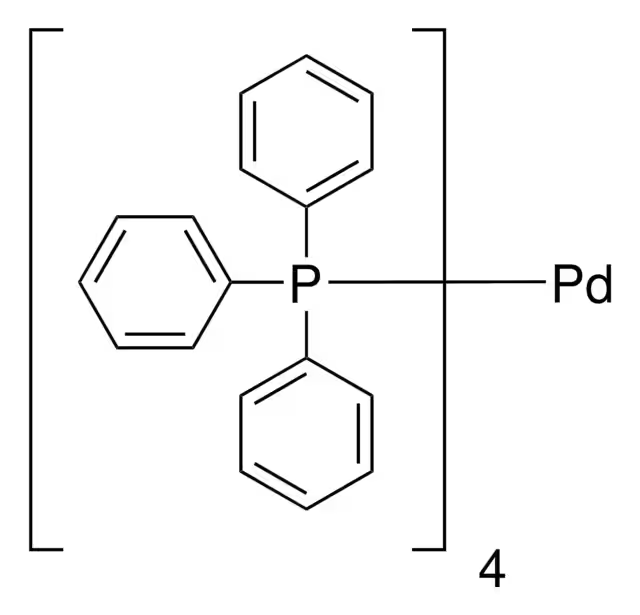
ARL7741 14221-01-3

ARL8576 51364-51-3

ARL7736 53189-26-7

ARL9913 35138-22-8
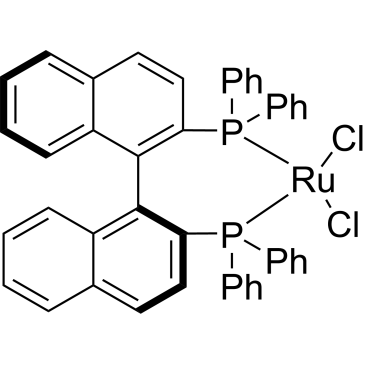
ARL9914 134524-84-8

ARL9089 12354-85-7
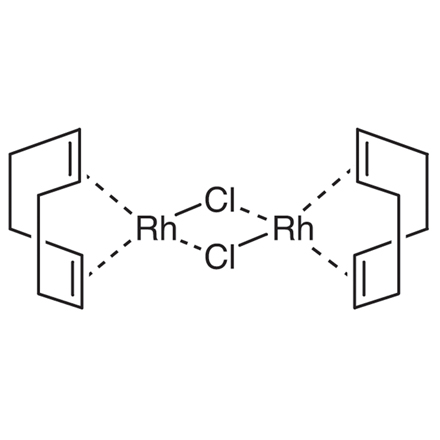
ARL6217 12092-47-6
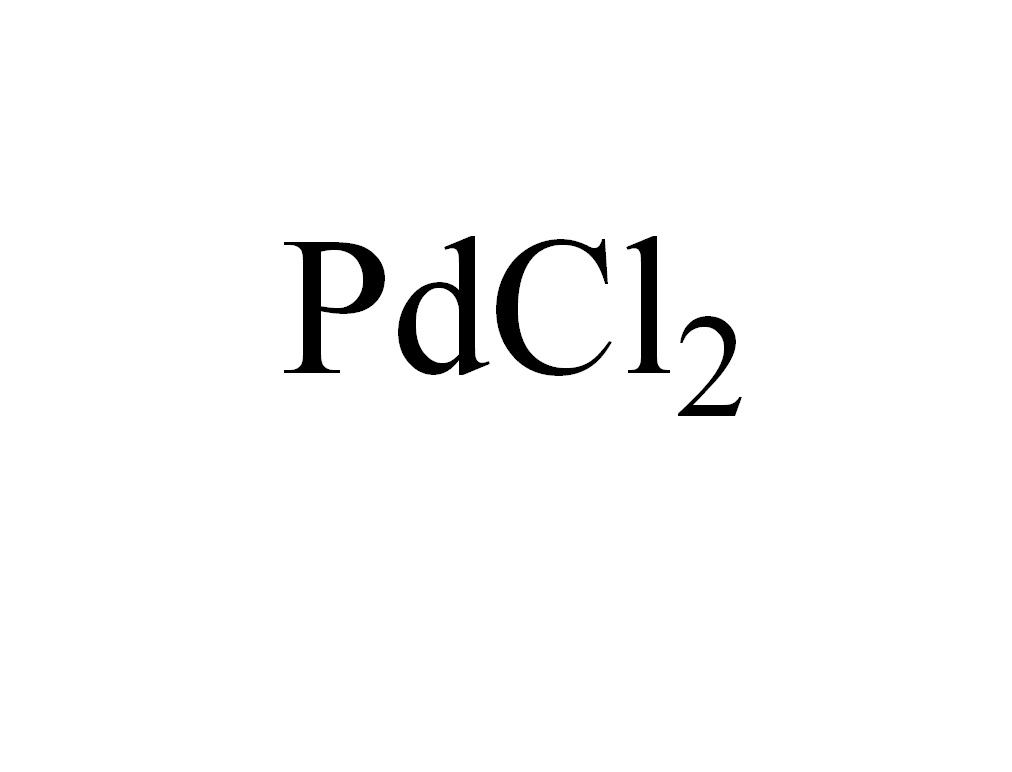
ARL7730 7647-10-1
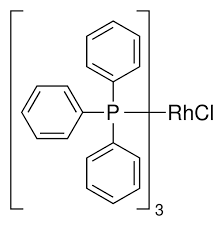
ARL6527 14694-95-2

ARL7732 7440-05-3

ARL7731 7440-05-3

ARL8821 12135-22-7
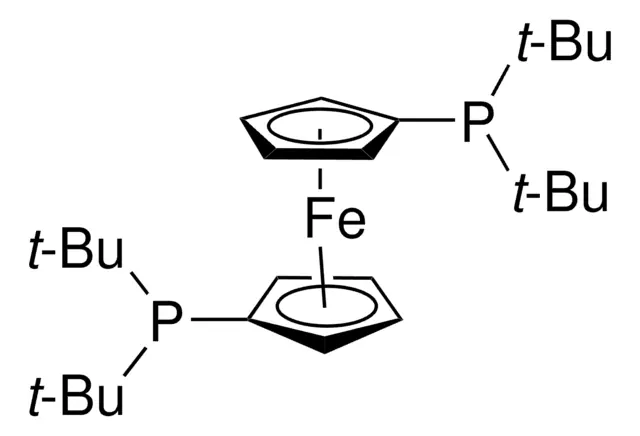
ARL4326 84680-95-5
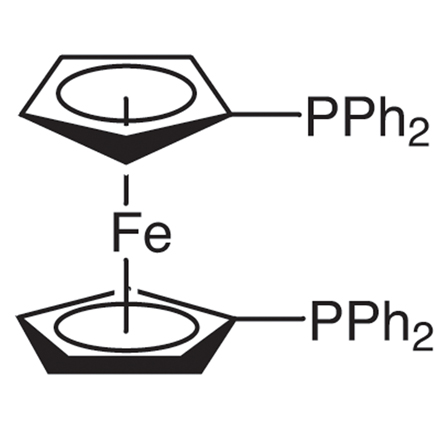
ARL4723 12150-46-8

ARL1062 14264-16-5

ARL8119 2923-28-6
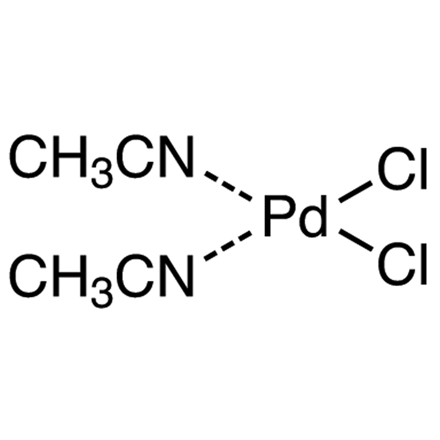
ARL1050 14592-56-4

ARL9928 114757-66-3

ARL7869 1314-15-4
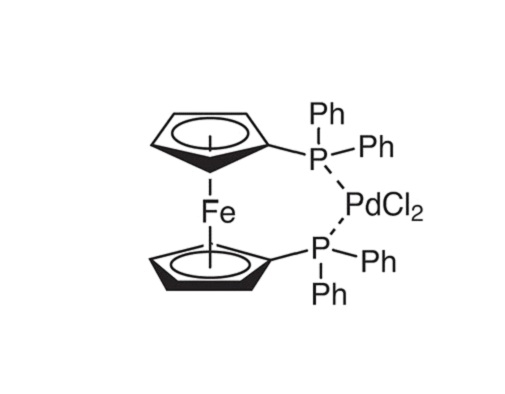

ARL6290 142-71-2

ARL10104 3375-32-4

ARL1051 14220-64-5
ARL10184 7440-05-3
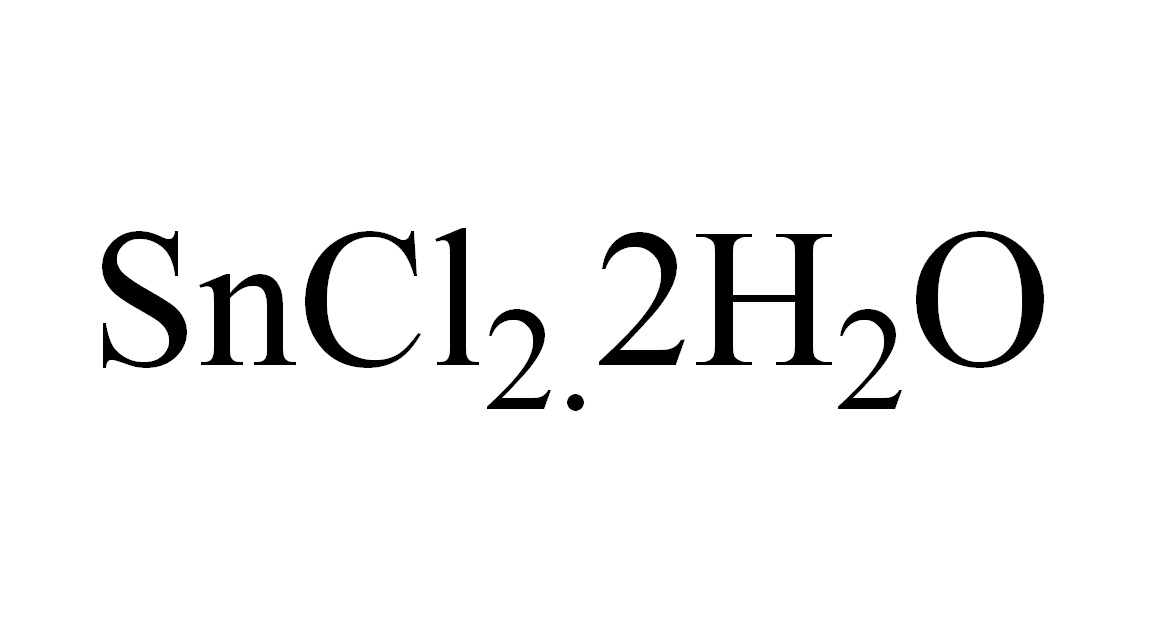
ARL10201 10025-69-1

ARL10203 18282-10-5
This is a sample COA and may not represent a recently manufactured lot of the product.